How is hydrogen produced?
While several methods for producing energy-ready hydrogen exist or are under development today, each exhibits a combination of economic, technical, safety and environmental advantages and drawbacks that must be considered for effective production.

Tóm lại
- There are many ways to produce hydrogen, each varying in technical, financial and environmental viability.
- Hydrogen exhibits high energy density by mass and clean burning potential, but the lack of robust production and storage infrastructure must be addressed to enable widespread adoption.
- Grey and blue hydrogen, produced from natural gas through steam methane reforming or autothermal reforming, are currently the most common types within industry.
- Turquoise hydrogen, produced by methane pyrolysis, separates natural gas into hydrogen gas and solid carbon at high temperatures, prompting easier carbon capture than in a gaseous state.
- Green hydrogen splits water into oxygen and hydrogen gas using electrolysis, powered by renewable energy sources.
- Less common methods for hydrogen production include nuclear-assisted, sunlight-powered photocatalytic, biological and biochemical approaches, all of which are in early-stage development.
Production considerations
As industry adds sustainable energy sources in the global fight against climate change, hydrogen is emerging as a clean and versatile alternative to fossil fuels. However, realizing this fuel’s potential hinges on developing and deploying efficient, cost-effective and environmentally responsible production methods.
Hydrogen’s non-competitive price per unit of power generated compared to conventional fossil fuels remains one of its most significant drawbacks, restraining it from widespread adoption. As a result, tax credits and other governmental incentives are responsible for the hydrogen economy’s evolution to a large degree, as these help offset costs associated with production and utilization.
There is a wide range of production methods available for hydrogen, each varying in technical, financial and environmental viability. This page provides an overview of common production methods, along with a few experimental methods that are still being developed.
Chemical characteristics
Hydrogen provides a compelling range of technical advantages as an energy carrier, including:
- High energy content per unit mass compared to conventional fuels
- Potential for zero carbon emissions at the point of use when consumed in a fuel cell
- Lack of energy degradation when stored long-term, a significant advantage compared to batteries
- Versatility across various applications, including transportation and energy storage
However, challenges remain for widespread adoption in industry, primarily centered around available infrastructure and cost.
Compared to unleaded gasoline, hydrogen is energy-dense by mass, but not by volume. By mass, it exhibits an energy density around three times that of gasoline, making it attractive for applications where weight is critical, such as long-range transportation.
However, its low volumetric density necessitates additional considerations for storage, often consisting of pressurization for gaseous hydrogen, or liquefaction through cryogenic techniques. Although these methods increase density, they introduce operational complexities and require energy consumption to alter and maintain hydrogen in its controlled state, which requires specialized infrastructure. Furthermore, its flammable nature - and propensity for leaks due to its small molecule size - demands stringent safety protocols throughout the value chain.
Grey and blue hydrogen
Grey hydrogen, the most common type currently encountered in industry, relies on either of two thermochemical processes: steam methane reforming (SMR) and autothermal reforming (ATR).
Both SMR and ATR begin with a hydrocarbon feedstock, typically natural gas, that consists primarily of methane (CH4). In SMR, this methane is preheated and combined with high-temperature steam (H2O) in the presence of a catalyst within a reformer unit. ATR introduces both steam and a controlled volume of oxygen gas (O2) within the reformer unit, which causes combustion. Unlike SMR, ATR does not require external heat for the methane reforming process.
Under extreme temperature conditions in either process, the catalyst facilitates dissociation of the methane and water molecules in the reformer unit, breaking their chemical bonds. This thermal cracking process results in a product gas stream containing the desired hydrogen, along with carbon monoxide and trace amounts of carbon dioxide. The carbon gases are typically detained by adsorbent beds just downstream of the reformer, while hydrogen flows through the chamber where it can then be stored and used on demand later.
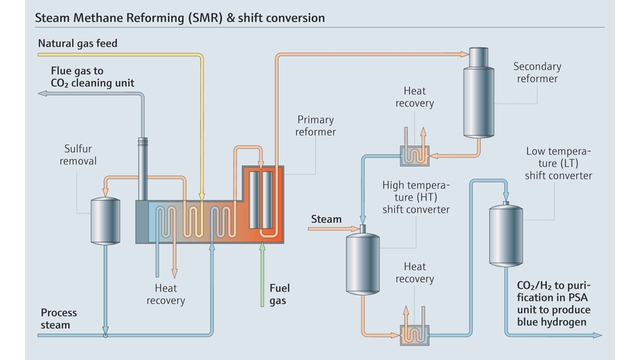
Steam Methane Reforming process and shift conversion.
In applications where carbon dioxide is released into the atmosphere, the hydrogen produced is termed “grey hydrogen.” If the CO2 instead sequestered, the hydrogen becomes “blue.”
ATR is more energy-efficient than SMR because it does not require an external heat source. In addition, the controlled dosing of oxygen in the reformer unit reduces carbon monoxide output substantially, producing a purer stream of carbon dioxide than SMR. This makes it ideal for blue hydrogen production. However, ATR is more complex to monitor and control, especially with respect to combustion, a process with significant safety concerns.
Insights
The controlled dosing of oxygen in an ATR reformer unit produces a purer stream of carbon dioxide than SMR, making it ideal for blue hydrogen production.
The environmental viability of blue hydrogen hinges on the effectiveness and scalability of CCS technologies, which remain areas of ongoing research and development.
Turquoise hydrogen
Turquoise hydrogen is produced using methane pyrolysis, where natural gas is heated directly to extreme temperatures - greater than 900°C (1652°F) - at which point it breaks down into hydrogen gas and solid carbon. The carbon byproduct in solid form is captured more easily than in the gaseous phase.
When the heat required for pyrolysis is generated from renewable sources, such as solar or geothermal, turquoise hydrogen becomes cleaner. Although this production method shows promise, it is still in the initial stages, requiring larger-scale demonstrations to prove its viability and ensure the captured carbon can be permanently stored.
Green hydrogen
Green hydrogen is regarded as the gold standard of sustainable hydrogen, produced from renewable - such as solar, wind, or hydro - energy via water electrolysis.
Electrolysis is a process that splits water molecules (H2O) into hydrogen (H2) and oxygen (O2) using electrical energy. An electrolyzer consists of two electrodes - an anode and cathode - and an electrolyte, which is a conductive solution that facilitates the flow of ions between the electrodes.
When direct current electricity flows through the system, reduction occurs at the cathode, which takes on electrons. This attracts negatively charged anions from the electrolyte to fill the void left by cathode-attracted electrons. Oxidation occurs at the anode, releasing electrons and causing positively charged cations from the electrolyte to migrate toward it.
At the cathode, positively charged hydrogen (H+) atoms gain electrons and form hydrogen gas, while at the anode, water molecules lose electrons, releasing oxygen gas and replenishing hydrogen ions moving toward the cathode.
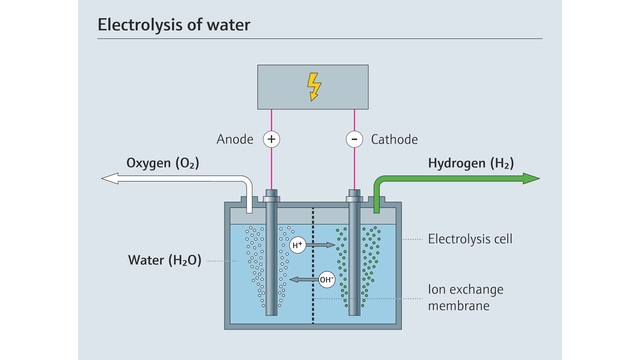
Renewable energy-powered electrolysis of water is used to produce green hydrogen.
The net result is the separation of water into hydrogen and oxygen gas molecules. This green hydrogen is stored, while the oxygen can be released into the atmosphere without any harm.
When excess renewable energy is available, green hydrogen provides a sustainable way of harvesting it to supply the grid later when needed. Unlike power stored in batteries, stored hydrogen power does not degrade over time, rendering it useful especially for seasonal or long-term energy storage.
Insights
Unlike power stored in batteries, stored hydrogen power does not degrade over time, rendering it useful especially for seasonal or long-term energy storage.
Hydrogen is an effective long-term storage mechanism for renewable energy, which is often limited to production during certain times and seasons.
However, the laws of thermodynamics dictate that the energy required to power electrolysis for the production of hydrogen is greater than the energy available from the product. Current estimates by the National Renewable Energy Laboratory indicate electrolysis is approximately 70-80% efficient, meaning that portion of the renewable energy input to execute the process is available as potential energy in the resulting hydrogen.
Additionally, electrolyzer infrastructure is in its infancy, requiring further development and efficiency enhancements before its use can become widespread.
Less common methods
There are a few less-commonly employed means of producing hydrogen, including nuclear-assisted, photocatalytic water splitting and biological and biochemical methods.
Nuclear-assisted hydrogen production
Nuclear-powered electrolysis is a potential pathway to large-scale and carbon-free hydrogen production - referred to as “pink hydrogen” - although this method is still emerging. Because nuclear power plants operate continuously, they provide a stable energy source for hydrogen production, addressing the intermittency challenges associated with renewable energy. However, public concerns surrounding nuclear safety, waste disposal and the potential for proliferation are barriers to adoption.
Photocatalytic water splitting
Harnessing the power of the sun directly, photocatalytic water splitting utilizes semiconductor materials that absorb sunlight to split water molecules into hydrogen and oxygen without electricity. When photons strike a photocatalyst semiconductor, it excites electrons, which provide the energy to drive a chemical reaction, mimicking photosynthesis in plants.
This method is far from ready for mass rollout and further research is required to develop cost-effective photocatalytic materials. However, early trials indicate its efficiency is much higher than electrically-powered electrolysis.
Biological and biochemical hydrogen production
Another niche production possibility for future usable hydrogen is biophotolysis, which leverages the photosynthetic capabilities of algae and cyanobacteria in nature to produce hydrogen from bodies of water. Additionally, enzymatic reactions may be able to catalyze hydrogen production from biomass or water.
These methods are entirely experimental today, but exploring the limits and possibilities of hydrogen production is important for growing the hydrogen economy as an efficient and viable cornerstone for reducing industry-generated greenhouse gases.
Leveraging production methods effectively
Producing and using hydrogen effectively requires weighing financial, technical and environmental factors to drive decision-making. Refinement of and an increase in various hydrogen production methods will improve viability of hydrogen in many different applications.
Although grey hydrogen production using SMR or ATR is most prevalent currently, governmental tax incentives are increasing blue hydrogen production, which employs carbon capture technologies to mitigate environmental impacts. Green hydrogen, produced via renewable energy-powered electrolysis, presents a more sustainable solution, but its scalability and cost-effectiveness require further technological advances.
Emerging methods like methane pyrolysis and photocatalytic water splitting offer promising alternatives, but they are still in their nascent stages, requiring further research and development. A multifaceted approach that embraces a diverse portfolio of production methods, coupled with supportive policies and continuous innovation, is essential for driving hydrogen toward its potential as a sustainable energy cornerstone.



